E. coli Expression

Rapid, economical production of recombinant proteins in E. coli. This expression system is most suitable for proteins not requiring complex post-translation modifications for proper folding and function.
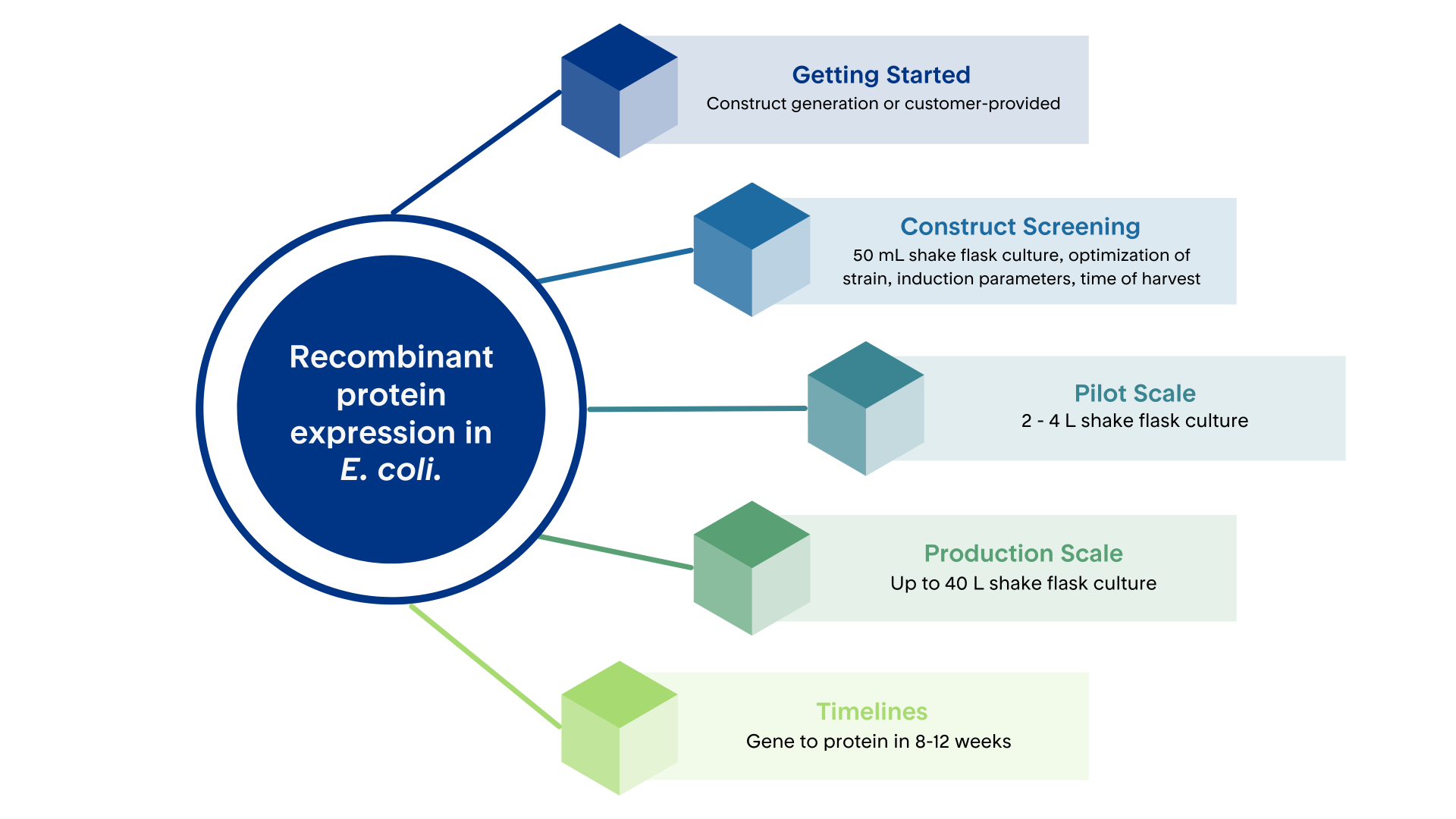
Proteos specializes in rapid, cost-effective, U.S.-based production of high-quality recombinant proteins using bacterial expression in E. coli. This system is ideal for expressing proteins of prokaryotic origin or eukaryotic proteins that do not require complex post-translation modifications for proper folding and functionality
Our protein production workflows are fully customizable and can include small-scale (50 mL) expression testing to evaluate and optimize key variables such as host strain selection, media composition, and induction parameters. This early-stage screening enables the identification of conditions that minimize cytotoxicity and improve soluble protein yield. Once optimal conditions are established, expression can be scaled to up to 40 L batch size using fully validated protocols, ensuring consistent and reproducible protein production in shake flask culture.
Production scales range from 50 mL to 40 L, and expression can typically be completed in 2–3 weeks as part of a streamlined 8–12 week gene-to-protein workflow.
Fusion proteins, such as maltose-binding protein (MBP) and glutathione S-transferase (GST), offer several benefits when expressing recombinant proteins in E.coli:
Overall, fusion proteins are a powerful strategy for increasing expression success rates and simplifying purification in prokaryotic system.