Protein Analytics

Customizable protein analytics packages to meet your specific needs.
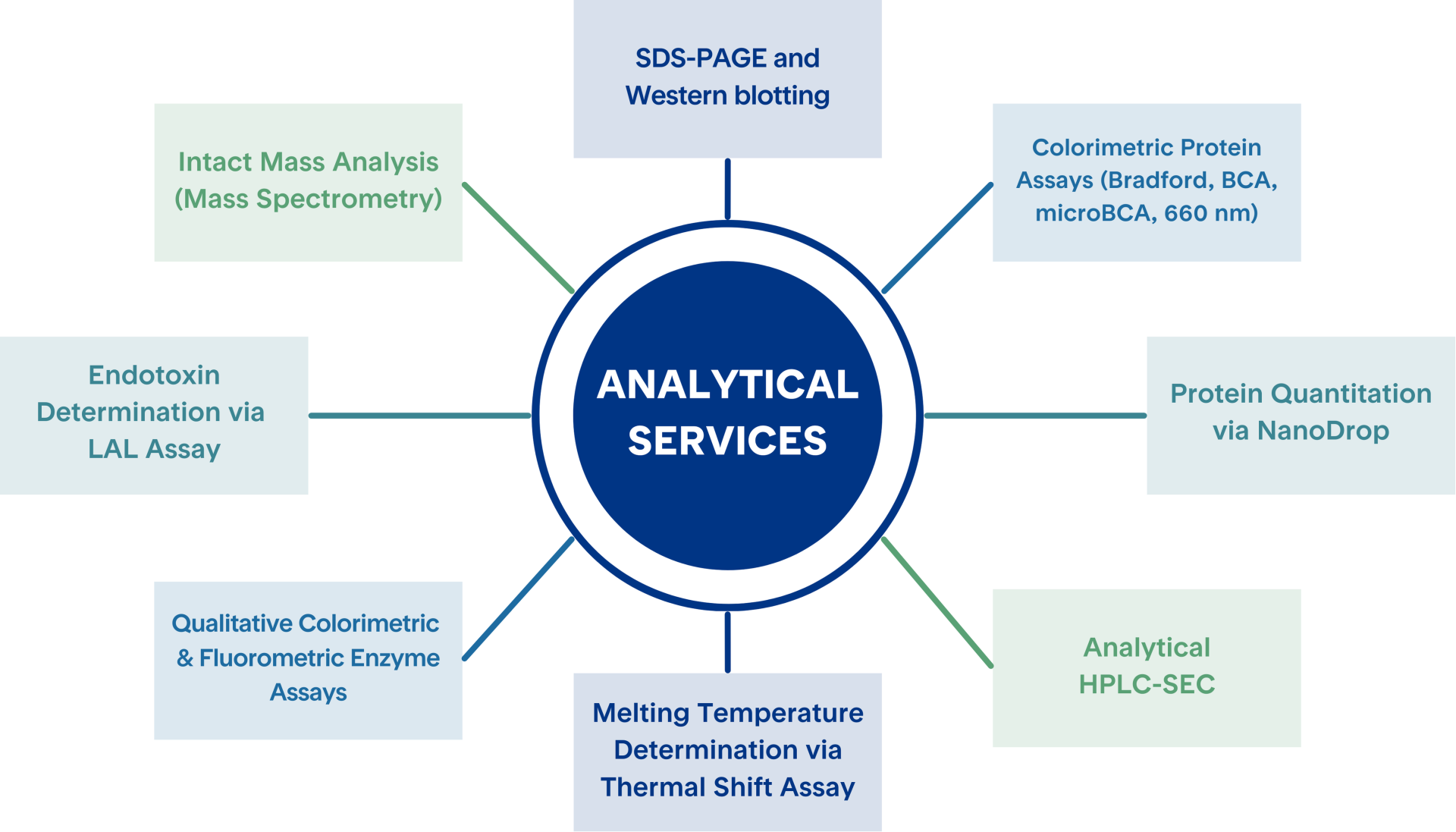
Our Protein Analytics platform offers a full suite of methods to characterize, quantify, and evaluate protein samples across multiple dimensions. Analytics packages are fully customizable to best suit your applications. Below is an overview of the key analytical techniques we offer.
High-resolution protein separation and immunodetection for molecular weight estimation and confirmation of protein identity.
Sensitive detection using BCA, Bradford, or other methods for accurate protein concentration measurements.
Rapid, small-volume protein concentration analysis based on absorbance at 280 nm.
Size-Exclusion Chromatography (SEC) for protein purity, aggregation, and oligomerization state determination.
Evaluate protein thermal stability and binding interactions through differential scanning fluorimetry (DSF)
Click here for additional information about Thermal Shift Assay analysis.
Assess enzymatic activity using commercially available kits to measure substrate conversion with visible or fluorescent signal readout.
Quantitative detection of endotoxin levels using the Charles River’s Endosafe® PTS system that utilizes Limulus Amebocyte Lysate (LAL) for rapid endotoxin testing. The Endosafe® PTS™ is FDA-licensed and meets the requirements of USP<85> and Ph. Eur. <2.6.14> for LAL testing.
Rapid and reliable intact mass analysis of proteins using the Waters BioAccord LC-MS system. Click here for additional information about Intact Mass Analysis.
We perform standard analytical testing on all recombinant proteins but cannot guarantee that a protein will be functional in your specific experimental system. Functional performance may vary depending on the application, formulation, assay conditions, and any downstream modifications.
A standard protein analytics package includes: