Antibody Production

Recombinant expression and purification of full-length IgG and antibody fusion proteins. Our comprehensive production capabilities encompass construct design, transient mammalian cell expression using in-house or customer-provided expression vectors, and affinity purification.
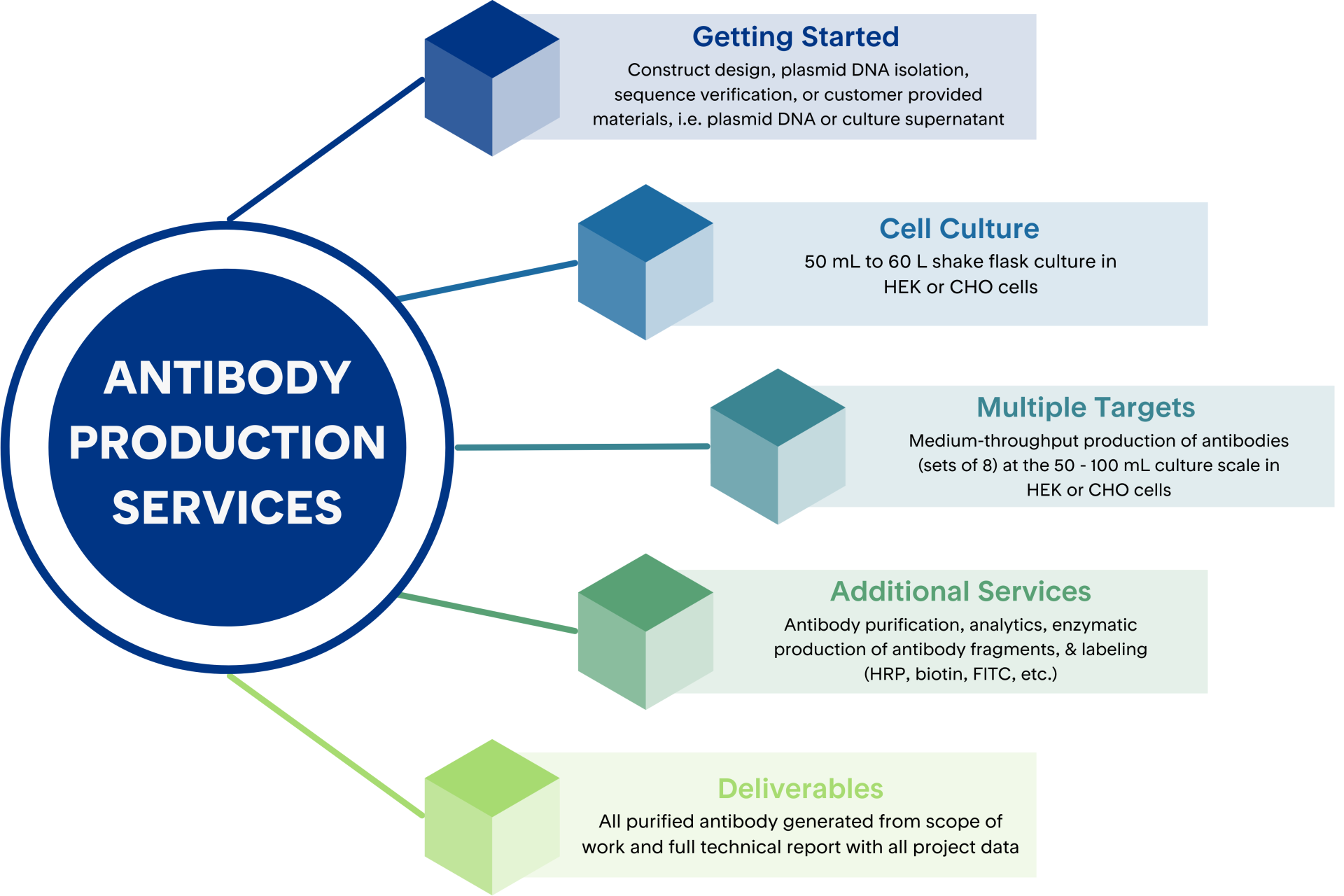
Our antibody production platform is designed for flexibility, scalability, and precision. Whether you’re working with full length IgG, engineered antibody formats, or antibody fusion proteins, we offer tailored workflows to meet your specific needs.
We deliver consistent, reproducible results with fast turnaround using optimized, validated protocols for recombinant expression and purification. Research scale, serum-free antibody production is available in shake flask volumes ranging from 50 mL to 60 L. Using a streamlined gene-to-protein workflow, recombinant antibody production is completed in 8–12 weeks.
Our industry-leading transient expression platform uses fully licensed HEK and CHO cell lines engineered to express Epstein-Barr virus (EBV) nuclear antigen. When transfected with constructs containing the EBV origin of replication (OriP), these cells support high-level episomal plasmid amplification and yield robust recombinant antibody expression. Mammalian cell expression systems provide the essential post-translational modifications needed for proper folding, efficient secretion, and full biological activity—making them the preferred choice for producing functional antibodies.
High-specificity affinity purification using Protein A, Protein G, or other antibody-selective affinity chromatography resins, followed by size exclusion chromatography (SEC). Our team provides expert support in optimizing resin selection, column configurations, buffer systems, and elution conditions to maximum purity and yield across a range of mAb purification workflows.
Our customizable protein analytics services include protein quantitation via absorbance at 280 nm using a NanoDrop, reducing/non-reducing SDS-PAGE, western blotting, analytical HPLC-SEC (pre- and post- freeze/thaw), intact mass analysis, endotoxin determination via LAL assay, and thermal shift profiling.. These methods enable thorough characterization and accurate quantitation of antibody samples to ensure quality and batch to batch consistency.
No, we do not produce polyclonal antibodies. We focus exclusively on recombinant monoclonal antibodies (mAbs), which provide superior consistency, specificity, and reproducibility across batches.
Antibodies and antibody-fusion proteins produced at Proteos are research-grade and are suitable for drug screening, assay development, and pre-clinical in vivo studies.