Recombinant Antigens, Methods for Determining Protein Quality: Part 5
Ensuring Excellence: Methods for Assessing Recombinant Antigen Quality
The production of recombinant antigens involves numerous considerations, such as construct design, affinity tags, expression system, purification scheme, formulation, and characterization. Among these, rigorous quality assessment is vital to ensure the antigen’s suitability for downstream applications. Below, we highlight key methods to evaluate recombinant antigen quality prior to immunization.
Visualizing Purity and Structural Integrity
SDS-PAGE combined with protein gel staining is a foundational method for assessing the purity of recombinant antigen preparations. Comparing reduced and non-reduced samples can also reveal the presence of disulfide-linked aggregates, providing insights into the structural integrity of the protein.
Analyzing Aggregation and Stability
Analytical HPLC-SEC is a powerful technique for evaluating sample purity and aggregation states under native conditions. Additionally, analyzing pre- and post-freeze/thaw samples helps identify potential aggregation risks that could compromise antigen quality during immunization.
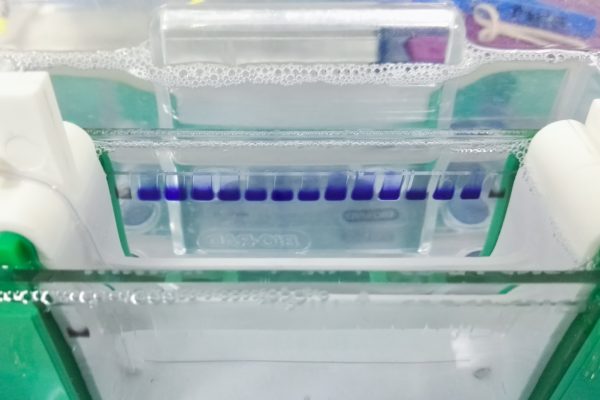
Ensuring Safety with Endotoxin Testing
The Limulus Amebocyte Lysate (LAL) assay is indispensable for quantifying endotoxin levels in recombinant antigen samples. Maintaining endotoxin levels within acceptable limits ensures the antigen’s safety for in vivo applications, preventing pyrogenicity and immune response complications.
At Proteos, we employ these and other reliable quality control measures to guarantee the highest standards for recombinant antigen production. Our meticulous approach ensures your antigens are ready for use in antibody production and other critical drug discovery applications.
Partner with Proteos to elevate your research. Contact us to learn more about our customizable solutions for recombinant protein production and quality assessment.